Nov 2025
OUTREACH is an open-label, multicenter, phase 2 study primarily evaluating the safety of Breyanzi as third-line or later treatment in patients with R/R LBCL at community medical centers in the United States across outpatient and inpatient settings.
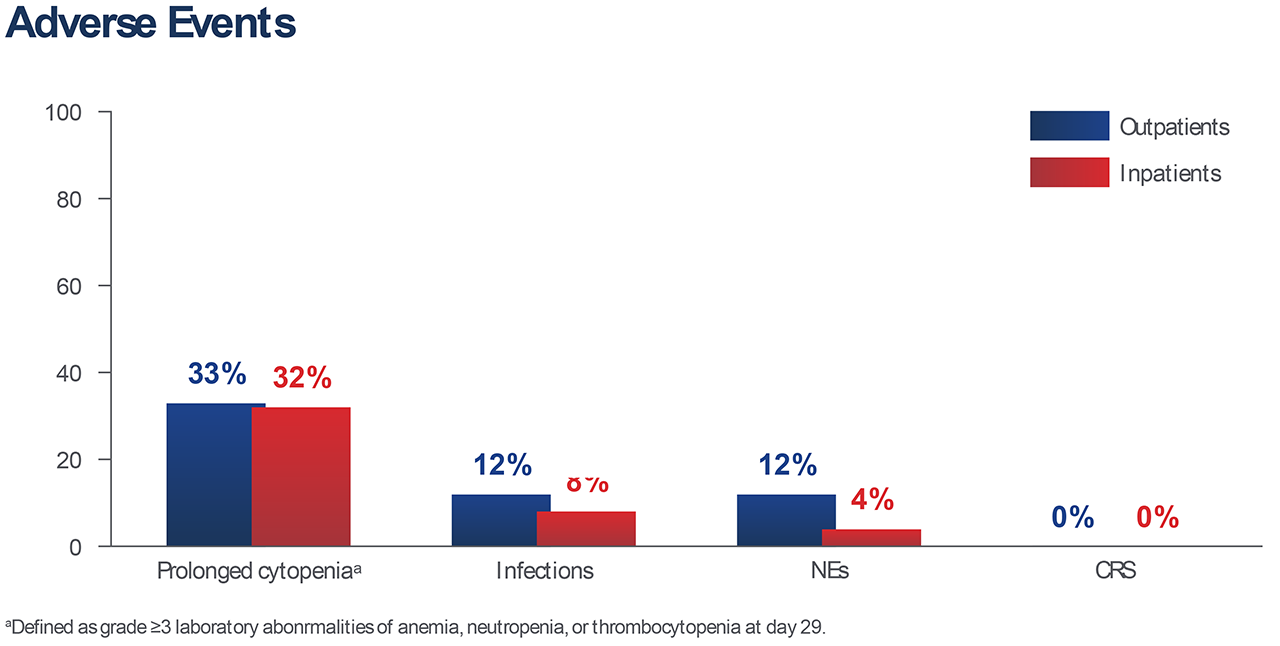
At a median follow-up of 10.6 months, the rates of grade ≥3 adverse events were as follows: CR.S, 0% in both outpatients and inpatients; neurologic events, 12% and 4%; infections, 12% and 8%; and prolonged cytopenia, 33% and 32%, respectively.
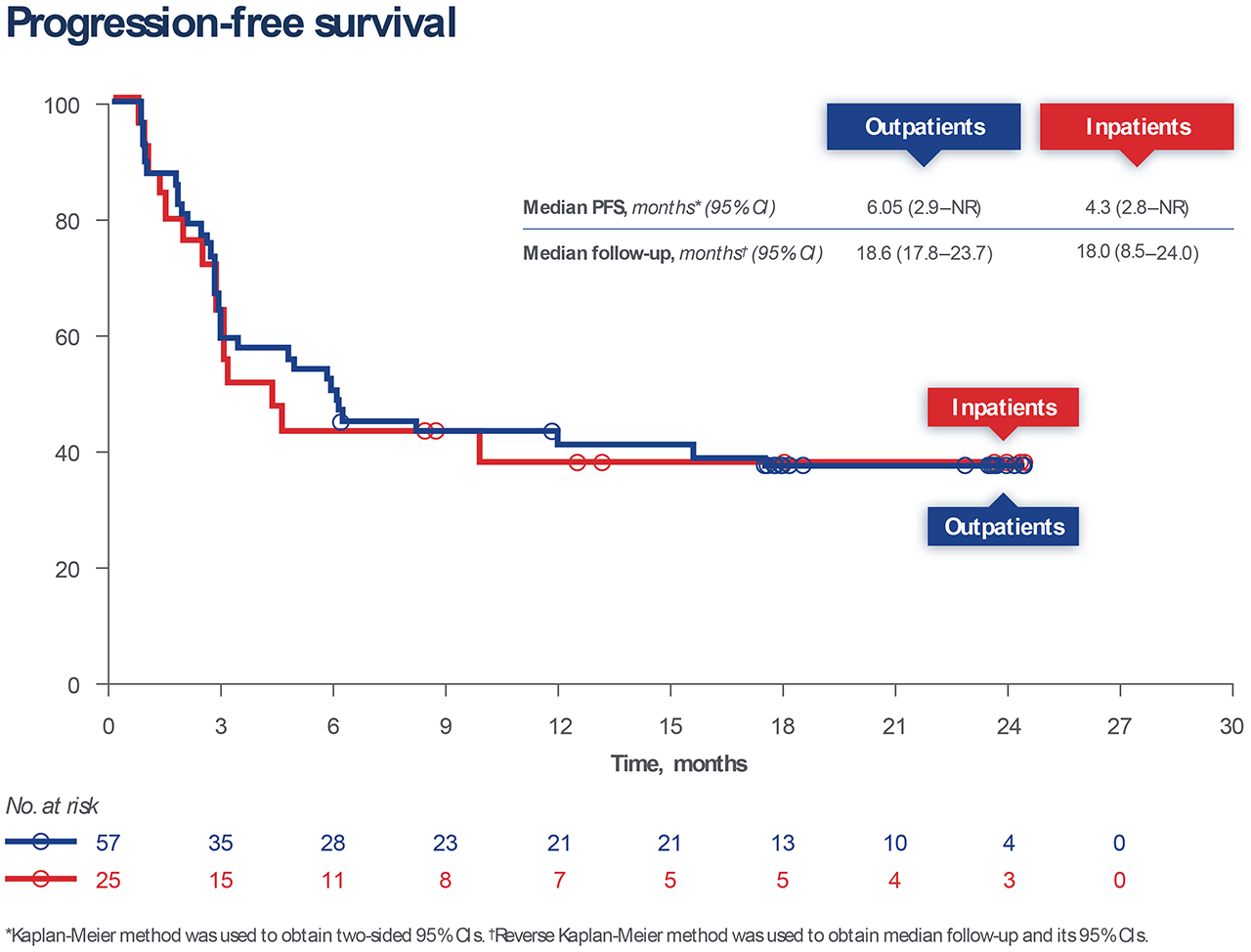
Among all Breyanzi-treated patients, the overall response rate (ORR) was 80% (n = 66;95% CI, 70.3–88.4), and results were similar between outpatients (82%; 95% CI, 70.1–91.3) and inpatients (76%; 95% CI, 54.9–90.6). Progression-free survival (PFS), a secondary outcome, was also comparable between inpatient and outpatient groups, as shown below.